
As a high speed electron approaches an atom, it will interact with the negative force from the electrons of the atom, and it may be slowed or completely stopped. In the Bremsstrahlung process, a high speed electron traveling in a material is slowed or completely stopped by the forces of any atom it encounters. X-rays produced by Bremsstrahlung are the most useful for medical and industrial applications.Īfter interacting with the atom, the free electron loses energy in the form of an X-ray photon What is bremsstrahlung?īremsstrahlung is a German term that means "braking rays." It is an important phenomenon in the generation of X-rays. One is called Bremsstrahlung and the other is called K-shell emission. There are two different atomic processes that can produce x-ray photons. The energy given up by the electron during this interaction appears as electromagnetic energy known as X-radiation. X-rays are generated when free electrons give up some of their energy when they interact with the orbital electrons or nucleus of an atom.

Why do we need electrons to produce x-rays? We need to have a source of electrons, a means of accelerating the electrons at high speeds, and a target material to receive the impact of the electrons and interact with them. To generate X-rays, we must have three things. X-rays can be produced in parcels of energy called photons, just like light. Roentgen discovered X-rays in the late 1800's while working with a cathode tube in his lab. You will recall from the history section that W.C. Where as gamma radiation is one of the products of nuclear decay of radioactive elements, X-rays are produced in high voltage electron tubes. As mentioned earlier, another type of radiation commonly utilized is X-radiation. So far our discussion has been primarily centered around radioactive elements, the structure of the atom, and the phenomenon of radioactivity.
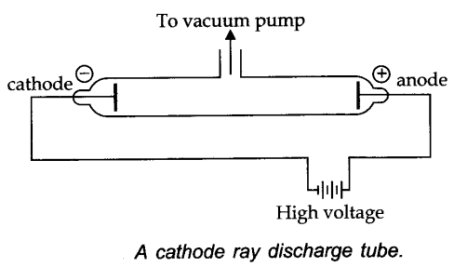
When cathode rays hit from the cathode travel towards the anode and hit the anode, a fluorescence or glow is produced.Ĭathode ray deviates from its path due to the application of an electric field. When a high voltage is applied between the two electrodes of an evacuated discharge tube and the back of anode of the discharge tube is coated with a material, like zinc sulfide. They were later named electronsafter particles postulated by George Johnstone Stoney. Thomson measured the weight of cathode rays and showed that they were actually a beam of particles.

German scientists Eilhard Wiedemann, Heinrich Hertz and Goldstein said they were some new form of electromagnetic radiation. Scientists Crooks and Arthur Schuster said they were electrically charged atoms. Scientists came up with two theories regarding cathode rays when they were originally discovered. Eugene Goldstein was the one who actually gave cathode rays their name. Cathode rays were first identified by a German physicist named Johann Hittorf when he realized that something was travelling through the tube.


 0 kommentar(er)
0 kommentar(er)
